
Baculovirus origin and history
Baculoviruses are a virus family which probably originated 400 to 450 million years ago and are ubiquitous in the modern environment (Heimpel et al. 1973). Apart from ancient Chinese literature, the earliest evidence of baculoviruses in Western literature can be traced to the sixteenth century by Marco Vida of Cremona describing gory liquefaction of silk worms (reviewed in Benz, 1986). Starting from the 1940’s baculoviruses were used and studied widely as biopestisides in crop fields (Miller, 1997). In the 1930’s a specific baculovirus from Finland was successfully introduced to Canada to abolish spruce sawfly, Gilpinia hercyniae (Arif, 2005; Balch & Bird, 1944). Since the late 80’s and 90’s they have been utilized as production of complex eukaryotic proteins in insect cell cultures (Kost & Condreay, 1999) and later on for viral display and gene therapy(Oker-Blom et al. 2003).
In 1985 it was discovered that baculovirus with suitable promoter was able to transduce mammalian cells (Carbonell et al. 1985), an observation confirmed not until 1995 (Hofmann et al. 1995). Even though baculoviruses are hindered by complement-system of blood (Hofmann & Strauss 1998), successful ex vivo experiments soon led to successful in vivo experiments in 2000 (Airenne et al. 2000). Since then, there have been several publications using baculoviruses with various targets in vitro and in vivo (Huser & Hofmann 2003; Kost et al. 2005).
The most studied baculovirus is Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV). This virus was originally isolated from lepidoptheran Alfalfa looper and contains a 134 kbp genome with 154 open reading frames (Ayres et al. 1994). The major capsid protein VP39 together with some minor proteins forms nucleocapsid (21 nm x 260 nm, (Fraser, 1986) which encloses the DNA with p6.9 protein. The virus appears in two distinctive forms depending on the stage of its lifecycle; a single budded virus (BV) and occlusion particle containing multiple virions, called occlusion derived virus (ODV) (see figure 1).
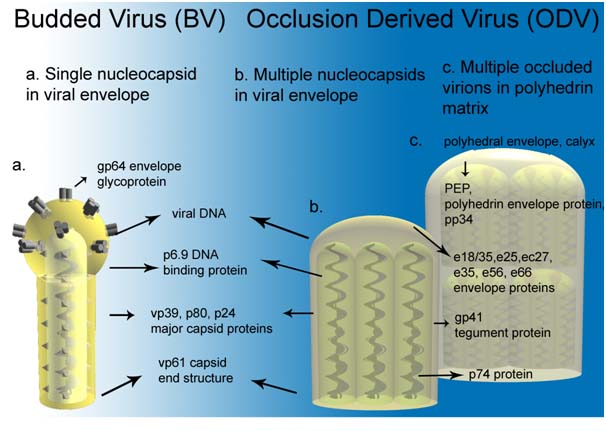
Figure 1: Baculovirus structural protein comparison between a) budded virus (BV) , b) occlusion derived virus (ODV) and c) polyhedra embedded virions (OB) (modified from (Blissard, 1996))
BV obtains its envelope from cell membrane and requires glycoprotein GP64 to be able to spread systemic infection. This protein is not found on ODV while several other proteins are only associated to ODV. Some differencies are also seen in the lipid composition of the viral envelope (Braunagel and Summers 1994).
Baculovirus trophism
It is not currently known if the baculoviruses have a specific receptor for their attachment and cell entry. However there are reports on specific requirements for their transduction (van Loo et al. 2001), involving heparin sulphate residues and electrostatic interactions. Additionally, interaction of Gp64 and cell surface phospholipids have been shown to play a role in the viral entry (Ernst et al. 2006; Tani et al. 2001). Interestingly, disruption of the cell-cell junctions with chelator EGTA has resulted to increased transduction efficiency (Bilello et al. 2001) achieved also by transient calcium depletion (Bilello et al. 2003). As it has been shown that basolateral surface is important for the baculoviral transduction, the loosening of cell-cell juctions might enable the entry from the basolateral side.
While the baculovirus receptor remains unknown, a large number of susceptible cell types suggests the viral tropism to be result from very universal interactions. However, when examing the difference between highly permissive and less permissive cell lines, it has been reported that the difference was in the presence or absence of baculovirus DNA in the nucleus (Barsoum et al. 1997), indicating that while the attachment might be universal, the later steps with nuclear entry and viral disassembly are likely to affect the baculovirus transduction and tropism (Kukkonen et al. 2003). Interestingly, baculoviruses are one of the few viruses which carry their capsid to the nucleus (van Loo et al. 2001), enabling the transport of therapeutic proteins to nucleus by using capsid display (Kukkonen et al. 2003).
The transgene expression of baculovirus is transient, peaking levels for 2-5 days in vitro (Liang et al. 2004) and from 3-5 days to one week in vivo (Airenne et al. 2000; Lehtolainen et al. 2002), but without complement lasting to nearly 200 days (Pieroni et al. 2001). However these results are with universal promoters and the transgene expression length and strength or tropism can be modified by using different promoters (Park et al. 2001; Wang et al. 2006; Wang & Wang, 2006).
Baculoviruses in Gene Therapy
Baculoviruses have several advantages as a gene therapy vector. Viruses have a long history with extensive studies on safety and viral structure (Black et al. 1997). They can be easily produced in high titers (up to 1012 pfu/ml), easily manipulated and quickly produced without animal serum (Luckow, 1993). Baculoviruses are not restricted to transduce dividing cells only, but can transducer G1/S arrested cells (van Loo et al. 2001). Most importantly, since viruses are derived from insect host, they do not replicate in vertebrate cells, however there is a contradictory report on expression of baculoviral immediate-early genes (Kenoutis et al. 2006). Still, the safety of the occluded viruses has been studied with various methods including intravenous, oral, intracerebral and intramuscular administrations in experimental animals and with feeding tests on voluntary human without any signs of toxicity (Heimpel and Buchanan 1967; Ignoffo and Heimpel 1965). Yet, very limited information is available on the effects of high dose of budded viruses in vivo (Raty et al 2008).
The rod-shape capsid enables high transgene capacity, without known limits (Cheshenko et al. 2001; Fipaldini et al. 1999; O'Reilly et al. 1994). However, the drawbacks include the production of virus in insect cells which results in the display of foreign glycoproteins, thus increasing possible immunogenic responses and inactivation by the blood complement system by classical pathway (Hofmann & Strauss 1998; Huser et al. 2001). This hindrance has been avoided by using complement-protecting factors (Hofmann et al. 1999; Huser et al. 2001; Pieroni et al. 2001), avoiding exposure to the complement (Airenne et al 2000; Sandig et al. 1996) or using the virus in immunopriviledged areas such as the eye (Haeseleer et al. 2001) and the brain (Lehtolainen et al. 2002; Sarkis et al. 2000). Currently the baculovirus has produced most promising results in vivo in the CNS gene delivery, for example inhibiting glioma cell growth in an animal model showing higher transduction rate in glioma as compared to surrounding brain tissue (Wang et al. 2006). Additionally,it has been reported baculoviruses may be able to utilize axonal transport to cell bodies (Li et al. 2004). While the later observation may be regarded as safety risk, together these studies may promote focusing the research to brain and CNS.
As compared to adenoviruses, the overall transduction efficiency of baculovirus is somewhat lower and while baculoviruses are less cytotoxic as compared to adenoviruses (Airenne et al. 2000; Lehtolainen et al. 2002), the baculovirus envelope is of insect origin, possibly creating immunoresponse with repeated administrations as suggested by cytokine eliciting interaction with Kupffer cells (Beck et al. 2000). Additionally, the large genome of baculovirus shows signs on unstability, hindering the production of established clinical gene therapy vectors (Pijlman et al. 2004).
Some of these drawbacks related to baculovirus properties can be avoided by enhancing transduction efficiency by pseudotyping (Kaikkonen et al. 2006) or by inserting enhancing elements to viral transgene cassette (Mahonen et al 2007) or by using histone deacetylase inhibitors (Condreay et al. 1999; Sarkis et al. 2000). Recently avidin-displaying baculoviruses, Baavi, (Raty et al 2004) have been used for biodistribution imaging with MRI and SPECT (Raty et al 2006; Raty et al 2007; Raty et al 2007) and magnetic targeting (Raty et al 2004).
An increasing number of baculoviral publications within gene therapy indicates that this vector is considered to be useful for gene and stem cell therapies and the development work towards first human trials continues.
About the Author
Jani K. Räty was born 1977 and finished his PhD from University of Kuopio, Finland 2006. Dr Räty has a decade long experience working with gene therapy vectors and various aspects of vector development and imaging. Besides vector development he's also an expert with GXP-IT issues and GMP consulting. Dr Räty is an editorial board member of Journal of Nanoneuroscience and besides study books has published several articles and book chapters about baculoviral gene therapy and imaging.
Further Reading
Balch, R. E. & Bird, F. T. 1944. A disease of the European Sawfly, Gilpinia hercyniae (HTG.), and its place in natural control. Sci. Agric. 65, 65-80.
Benz, G.A. 1986. Introduction: historical perspectives, p. 1?35. In: Biology of Baculoviruses, Volume I. R. R. Granados and B. Federici (Eds.). CRC Press, Boca Raton, Florida.
O'Reilly, D.R., Miller, L.K., & Luckov, V.A. 1994. Baculovirus expression vectors. A laboratory manual, 2 ed. New York, NY, Oxford University Press.
Miller, L.K. 1997. The Baculoviruses Plenum Press, New York
Black, B. C., Brennan, L. A., Dierks, P. M., & Gard, I. E. 1997, "Commercialization of Baculoviral Insecticides," In The Baculoviruses, L. K. Miller, ed., New York: Plenum Press, pp. 341-388.

































