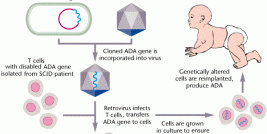
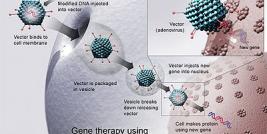
A recent article published on the New England Journal of medicine by Aiuti and colleagues reports on the progress of 10 patients that have been treated for ADA-SCID by gene therapy. This is a form of severe combined immuno-deficiency (SCID) where there is a lack of the enzyme adenosine deaminase (ADA), coded for by a gene on chromosome 20. The result is that the substrates for this enzyme accumulate in cells and as immature lymphoid cells of the immune system are particularly sensitive to the toxic effects of these unused substrates, the cells fail to reach maturity. Consequently, the immune system of the afflicted individual is severely compromised or completely lacking.
The authors of the current study report the four year follow-up on a group of patients that received transfusion of autologous CD34+ bone marrow cells transduced with a retroviral vector containing the ADA gene. The results show that 8/10 treated patients demonstrated sufficient re-constitution of their immune system to warrant the cesation of the standard enzyme-replacement therapy (the current standard of care in this disorder).
This is exciting news for the gene therapy field and extends on the initial success of the X-linked SCID trials, where gene transfer was demonstrated to fully re-constitute the immune system of boys suffering from this disorder. It is well known that the major setback in the X-linked SCID trial was the unfortunate development of leukemia from retroviral-transduced cells that was corellated with integration of the therapeutic gene near a specific locus. It remains to be seen whether a similar insertional mutagenesis event will occur in the ADA-SCID patients. But all indications thus far show that this is not the case and it has been argued that the mechanism of the ADA-SCID disease is sufficiently different that this is unlikely to occur.
In the past three years there has been an unprecedented increase in the number of gene therapy clinical trials and as the results of these trials start to accumulate, we can hopefully look forward to more success stories, which will hopefully boost the reputation of the gene therapy field.