Artcle by: Syamal Bhattcharya, MD; Zhiyong MI, PhD; Hongtao Guo, MD, PhD; Paul C. Kuo, MD
In recent years a novel crop of therapies called aptamers have been derived that take advantage of small segment nucleotides that tightly bind cell surface proteins. This interaction at the extracellular level impedes the normal cascade of the receptor protein, blunting or arresting the usual chain of intracellular events. In contrast to antibody directed therapies, aptamers are able to function at very low concentrations. In addition to the robust binding capability to target cell surface proteins (as described by the dissociation constant), added benefits of aptamer therapy include exquisite target specificity and a lack of immunogenicity (1, 2). When compared to antisense oligonucleotides, aptamers are superior in that the targets are extracellular.
Our group has been able utilize aptamers to impede the metastatic potential in MDA-MB231 breast cancer, as well as slow the local tumor 3-D growth rate. MDA-MB231 breast tumor cells use a secreted phosphoprotein, osteopontin (OPN), to mediate migration, local invasion, angiogenesis, cell colony formation, and metastatic potential. OPN has also been shown to protect cells from apoptosis (3). In a series of published reports, OPN is commonly secreted in metastatic breast, hepatocellular, and colorectal cancers (4). OPN functions by binding to cell surface receptors CD44 and agB3 integrin (5, 6, 7) initiating an intracellular cascade that include signal transduction mediators PIK3, JNK ½, SRC, and AKT. Matrix metalloproteinase-2 (MMP2) and uroplasminogen activator (uPA) signaling systems are also induced by OPN expression and cell surface binding. They function aside from physiologic roles in wound healing and tissue remodeling, they are vital in tumor progression by ezymatic degredation of extracellular matrices (8, 9, 10, 11).
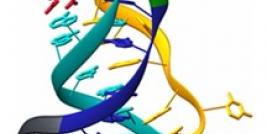
Using SELEX (systemic evolution of ligands by exponential enrichment), a large pool of potential ligands (> 1014) was employed to screen purified OPN binding (12). After eight rounds of binding and enrichment, a cohort of fifty potential ligands were isolated, of those twelve were found to be OPN-R3 aptamers with Kd of 18 +/- 0.2 nmol/l. There was no homology with the other thirty-eight candidates. OPN-R3 is a 40 nt ssRNA with a characteristic stem and loop structure. OPN to OPN-R3 binding was disrupted by the presence of OPN antibody. OPN to OPN-R3 binding must therefore depend on a specific active binding site. OPN-R3 was then mutated to define the active binding site- deletion constructs labeled OPN-R3-1, OPN-R3-2, and OPN R3-3 were studied with REMSA. It was evident that elements deleted in OPN-R3-2 and OPN-R3-3 are critical for OPN binding- these deletions prevented OPN binding. Forster resonance energy transfer (FRET) confocal microscopy with MDA-MD231 breast cancer cells was performed to see the effects of OPN-R3 inhibition of OPN cell surface binding in a series of incubations with OPN-R3, exogenous OPN, CD44 Ab, agB3 Ab, CD44 Ab and agB3 Ab, mutated OPN-R3, and no added substrate (control). OPN-R3, CD44 Ab and agB3 Ab, and agB3 Ab alone prevented FRET mapping indicating arrested OPN cell surface binding. Incubation with exogenous OPN (20nmol/l), and CD44 Ab yielded equivalent signals to control (19.6-24%). OPN-R3 successfully prohibited OPN binding to cell surface proteins.
Important mediators of the OPN dependant signaling pathway (PIK3, JNK ½, SRC, and AKT) were studies using western blots in MDA-MB231 after incubation with various substrates. Incubation with OPN-R3 inhibited expression of all mediators. agB3 Ab incubation inhibited PIK3, JNK ½, and SRC expression, in contrast to CD44 Ab incubation which inhibited JNK1/2, SRC, and AKT. This overlap may reveal a redundancy in the ultimate signaling pathway of CD44 and agB3 integrin binding of OPN. Mediator expression was not altered from control after incubation with exogenous OPN (20 nmol/l), mutant OPN-R3, and mutant OPN-R3 with RNase. Similar studies were performed to identify expression of MMP-2 and uPA, which are known to be regulated by OPN. Pro-MMP2, active MMP2, and uPA were examined. While OPN-R3 binding did not affect the expression of pro-MMP2, there was suppression of active MMP and uPA expression. Exogenous OPN, mutant OPN-R3, and RNase did not suppress or augment expression of the above proteins versus baseline from the MDA-MB231 cell line. OPN-R3 was shown to impede downstream signaling that follows OPN binding to the MDA-MB231 cell surface.
Effects of OPN-R3 binding to OPN on adhesion, migration, and invasion in the DMA-MB231 cell line was studied in Matrigel cell matrix. Matrigel is comprised mainly of laminin, collagen IV, heparin sulfate, proteoglycans, and entactin (13). To further simulate the extracellular matrix, transforming growth factor-B, fibroblast growth factor, and tissue plasminogen activator are included. OPN-R3 proved to temper all three factors: decreased adhesion by 60%, migration by 50% and invasion by 65%. Blockade of OPN function with agB3 Ab decreased respective factors by 30%, 40%, and 45%. CD44 Ab was also inhibitory by 40%, 30%, and 48% respectively. The percentages are in comparison to control assay with no added substrate. Other substrates tested- exogenous OPN, mutant OPN-R3, and RNase- did not affect adhesion, migration, or invasion.
In vivo studies required the modification of OPN-R3 to extend half life in human serum at 37oC. This was accomplished by 5’ cholesterol modification, inversion of 3’ deoxythimadine, and the addition of 2’-O-mthyl substituted nucleotides. These modifications increased the serum half life from eight hours to approximately 24 hours according to unpublished data from the manufacturer. Equivalent OPN binding of the modified OPN-R3 was confirmed with REMSA when compared to OPN-OPN-R3 interactions. MDA-MB231 cells engineered to produce luciferase were implanted into the mammary fat pads of female NOD SCID mice. Two groups of mice were in the treatment arm; treatment via mouse tail vein injections with modified OPN-R3 or mutant OPN-R3 every forty eight hours. Bioluminescence was monitored at day 10, 20, and 30. The luciferase signal of the modified-OPN-R3 treated mice was significantly decreased when compared to controls and mutant OPN-R3 treated populations. OPN-R3 treatment yielded a four-fold reduced signal at 20 days and twelve-fold reduction at 30 days. Tumor volumes were tracked daily noting an eighteen to twenty-fold reduction in tumor volumes at 20 days and eight-fold tumor volume reduction at 30 days compared to mutant OPN-R3 treated and control subjects. To characterize metastatic potential, lung tissue was examined. OPN-R3 treated subjects showed only 1% of the tumor burden found in mutant OPN-R3 and control lung tissue. It appears that OPN-R3 treatment effectively decreases local tumor growth and distant metastasis MDA-MB231 tumor cells in xenografts.
This “proof-of-concept” study shows that there may be a role for OPN-disrupting aptamers in the inhibition of MDA-MB231 breast cancer. If this mechanism of OPN mediated tumor growth and metastatic behavior continues to be applicable to other primary cancers, namely colorectal and hepatocellular, there is potential for expanding current treatment modalities. Arresting tumor growth and preventing metastatic behavior could expand the use of neoadjuvant modalities.