Florida Biologix’s primary focus is to produce, test and/or fill cGMP compliant biopharmaceutical clinical trial material on time and at a reasonable price. Our unique combination of assets allows us to meet your outsourcing needs quickly and cost-effectively; accomplishing your goal of moving biologics from bench to clinic.
Some quick facts:
- Our technical managers have over 100 years combined manufacturing and testing industry experience and have worked on over 90 successful INDs and multiple licensed commercial products
- Our scientists will work with you to ensure excellence in all phases of production, from developing a robust, scalable process through aseptic fill and finish, and product release
- We have a 23,000 square foot, state-of-the-art, validated, multi-product manufacturing and testing facility. An adjacent building houses our 5,000 square foot, fully-equipped, process development laboratories
- We are flexible enough to work within a client's timeline and budget
- Our in-house Quality Assurance team provides regulatory compliance oversight
- Our Quality Control laboratory provides a broad range of testing and assay development services
- Clients give Florida Biologix very high ratings on post-project satisfaction surveys
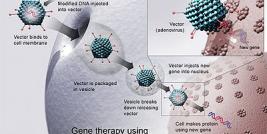
cGMP Compliant Vector and Vaccine Production
Our experienced and fully trained staff, using state-of-the-art disposable technologies, generate the highest quality bulk drug substance and finished product. The Florida Biologix manufacturing facility was designed and built to accommodate simultaneous production of multiple products. The facility has been commissioned and fully calibrated with all systems and equipment validated. Preventive maintenance, calibration and revalidation programs are in place, as are cleaning and changeover procedures. Back-up generators ensure all equipment is protected from power outages.
cGMP Master and Working Cell or Viral Banks
Prior to vector clinical lot manufacturing, Florida Biologix can produce either a Master/Working CELL bank (MCB and/or WCB used in vector and vaccine manufacturing) or a Master/Working VIRAL bank (MVB and/or WVB). These banks are made in spatially segregated, campaign-dedicated ISO 7 cleanroom modules. Once grown, vialed, tested, characterized, and frozen in controlled rate freezers, the banks are stored in validated, continuously monitored liquid nitrogen freezers. For additional protection of these valuable materials, offsite storage is also available.
Non-GMP production services are available for your research and non-clinical toxicology studies
cGMP Compliant Vector and Vaccine Production Modules
The ISO 7 modules are capable of handling BSL-1 or BSL-2 gene transfer agents. The modules have dedicated gown-in and degown rooms allowing for unidirectional flow of material and personnel, and single pass air supply with HEPA filtration at 60 air changes per hour. Our facility design, rigorous cleaning procedures and procedurally controlled flow patterns ensure that there is no cross contamination of any material from one module to another.
Upstream Cell Culture Processing
We can produce cGMP compliant cell culture supernatants or lysates containing viral vectors (in a 2 x 200 L stirred-tank disposable bioreactor, cell factories and/or Wave bioreactors). We have the technology for producing vectors by transient transfection for recombinant adeno-associated viral vectors or lentiviral vectors, infection of cell substrates with adenoviral, baculovirus or herpesviral vectors, and producer cell line culturing of retroviral vectors.
Downstream Purification and Processing
We have the capability and experience to purify vectors using column chromatography, filtration and centrifugal methods. We use state-of-the-art equipment to purify and further process the target vector.
- Cell lysis and clarification
- Chromatographic purification
- Diafiltration and other membrane filtration technologies
- Ultracentrifugation
- Formulation, fill, and finish
Clone Isolation and expansion
We can clone and expand viral seeds used in vector and vaccine manufacturing.
- Plaque purification
- Clone screening and lead clone identification
- Lead clone expansion and viral seed banking
In-House QC Analytical Methods Development
Our QC group develops, qualifies, and performs in-process, release, and stability testing.
- Infectious and vector genome titer determination
- Transgene expression assays
- Purity and identity testing