Article by: 696
The recent developments of viral based vectors in design, in biosafety and in accomplishing high transfection efficiency for transgene expression into target cells makes them attractive tool for various gene transfer applications. Several kinds of viruses, including HIV derived Lentivirus vectors (LVs), non-HIV based Retroviruses, Adenoviruses, Adeno-associated viruses, Baculovirus and Herpes simplex viruses have been manipulated for use in gene transfer and gene therapy purposes. Each viral vector system is integrated with a distinctive in built properties that affect its suitability for specific gene therapy purposes. Although LVs offered many unique solutions for advanced gene therapy research and clinical applications, however, several important issues of LVs gene therapy must be overcome before it gains widespread use. Nonetheless, the number of promising gene therapy studies in progress are highly encouraging and outcome from these clinical trials will provide valuable insights particularly for the clinical suitability and safety profile of LVs. This review contain a comprehensive discussion starting with the general background of the field thereafter discussing the salient features of recent developments in viral vector system in general and with special focus on LVs.
Key Characteristics of Viral Vectors
Viruses are obligate intra-cellular parasites, which typically transfect their own genetic material into the host cell extremely efficiently, however in the case of gene therapy vectors; the viral particles encapsulate a modified genome carrying a therapeutic gene cassette in place of non essential viral genome. Although these gene therapy viral vectors constructs, each feature differing mechanisms for targeting infection, and their ability to infect both dividing and non-dividing cell populations, however they all typically possess high rates of infectivity into most target cell. Since different viral vector systems have their own unique advantages and disadvantages, they each have applications for which they are best suited for (Table 1). Therefore, the aim of several ongoing and completed research studies involving these viral vectors had been to redefine their biosaftey, improvement in gene transfer methods, to provide efficient expression of desired transgenes (1-8). The great majority of current clinical gene therapy trials worldwide involve viral vectors derived from retroviruses (including MLV based), LVs (HIV based), adenoviruses, adeno-associated viruses, herpes viruses and alpha viruses (9-13).
Table 1: Gene Therapy Viral Vectors:
| Viral Vectors | Transgene Size | Host | Range |
|---|---|---|---|
| Adeno-Associated Virus | ~4kb | Dividing and non-dividing cells | Low long-term expression |
| Adenovirus | up to 30kb | Broad Range | Transient expression |
| Alphavirus | 8-10kb | Broad Range | Transient high expression |
| Herpes Simplex Virus | 40kb | Broad | Latent infection, long-term expression, low toxicity |
| Lentivirus | 8-10kb | Dividing and non-dividing cells | Chromosomal integration, long-term expression |
| Retrovirus | 8kb | Restricted to dividing cells | Chromosomal integration, long-term expression, insertional mutagenesis |
| Baculovirus | >20kb | Dividing and non-dividing cells | Chromosomal integration, transient expression in mammalian cells |
To design a viral vector, it is crucial to separate cis-acting sequences, required for the transfer of the viral genome to target cells from the trans- acting sequences encoding the viral proteins (14). Thus the biosafety and the efficacy of vector primarily depend on the level to which segregation of cis- and trans-acting functions of the viral genome is obtained. Typically, these viral constructs are then introduced in to a producer cells that would generate infectious but replication deficient viral particles, as these particles can only encapsidate, (that results in single round of infection, a process known as transduction). For most gene therapy applications, it is important that appropriate amounts of a therapeutic gene are delivered into the target tissue, at the same time it should not create substantial toxicity. In the past several years, there have been several clinical trials in which patients have benefited from gene therapy protocols (15-17), these progresses, however, have addressed many important biological issues, for instance, acute toxicity generated from the introduction of foreign genetic materials, cellular immune responses directed against the transduced cells, humoral immune responses against the therapeutic gene product and the potential for insertional mutagenesis with integrating type of viral vectors (18, 19). In general, the Integration process is well tolerated by most transduced cells in vitro however a potential problem with in vivo instillation of viral vectors is that the administered virus would not only infect desired targets, but multiple other cell types, which have a low likelihood of contributing to clinical effectiveness, might also be exposed. These unintended exposures to the cells with the viral vector could also possibly result in deleterious consequences, such as activation of the inflammatory response or insertional oncogenesis, if an integrating viral vector is used as seen in MLV based gene therapy trials (18, 20, 21). Additionally, it is important to address the transmission potential of vector sequences into germ cells as any possibilities of germline transmission raise important biosaftey concerns. In the last several years, different variations in vectors design have been introduced, such as self-inactivating vectors and vectors which have additional safety features or designing the integrating vectors in such a way so that it can integrate into pre-existing transgenic loci within the genome. Furthermore, improvements in safety may be also gained by using tissue-specific promoters to drive transgene expression, as it would not only be more tightly regulated expression, but also it would minimize the potential of insertional oncogenesis that are often associated with over expression strategies(22, 23).
Lentiviral Vector System
Lentiviruses comprise a genus of the Retroviridae family that includes the human pathogen Human Immunodeficiency Virus (HIV). The replication incompetent vector particles, LVs have been demonstrated to mediate stable and long-term transgene expression in various non-dividing and other difficult to transduce cells such as stem cells, nerve cells lymphocytes, and dendritic cells (23-25). Long-term transgene expression potential is of particular important for in vivo clinical management of chronic disease (9, 15, 23, 26). In addition, the LVs can be pseudotyped with specific viral envelopes that highly affect the vector tropism and enhanced biosafety and transduction efficiency. However though LVs are designed to be replication-defective, there are constant concerns about the possible generation of wild type or novel human pathogens, while using these vectors following clinical administration. Therefore significant modifications have been made in the LVs since the initial findings by Naldini and co-workers (27). Continual efforts are ongoing in recent years that have attempted to further delete wild-type HIV sequences in the lentiviral transfer (integrating) and packaging vector to enhance vector titer and transgene expression. The newer generation LVs technology has many in-built safety features that include separation of critical components of the viral genome onto distinct plasmids (split genome approach) that allows the safe production of one-round infectious viral particles. Besides, to delineate the mechanism by which a specific gene contributes to cell growth and viability, inducible and/or conditional systems were explored (28-30). Conditional gene expression is an absolute requirement in certain cases to have a tight regulation over transgene expression, for e.g. generation of a transgenic line may become problematic if the presence of the transgene leads to conditions which are toxic, lethal to cell growth or probably responsible for causing cell differentiations. For regulated gene transfer, most of the current approaches rely on drug-controlled molecular switches. The most used Tetracycline system (Tet) includes two basic variants namely, the Tet-Off system (tTA) and the newer version i.e. Tet-On system (rtTA) (Figure 1). Tet-on would require tetracycline for binding to the tetracycline-responsive promoter followed by activation of downstream genes while tet-off activates in absence of tetracycline. Tetracycline-dependent regulatory systems (Tet based systems) have been successfully employed during enormous biological studies, including transgenic mouse modeling and Induced pluripotent stem cell (iPSc) generations as discussed below (31-33).
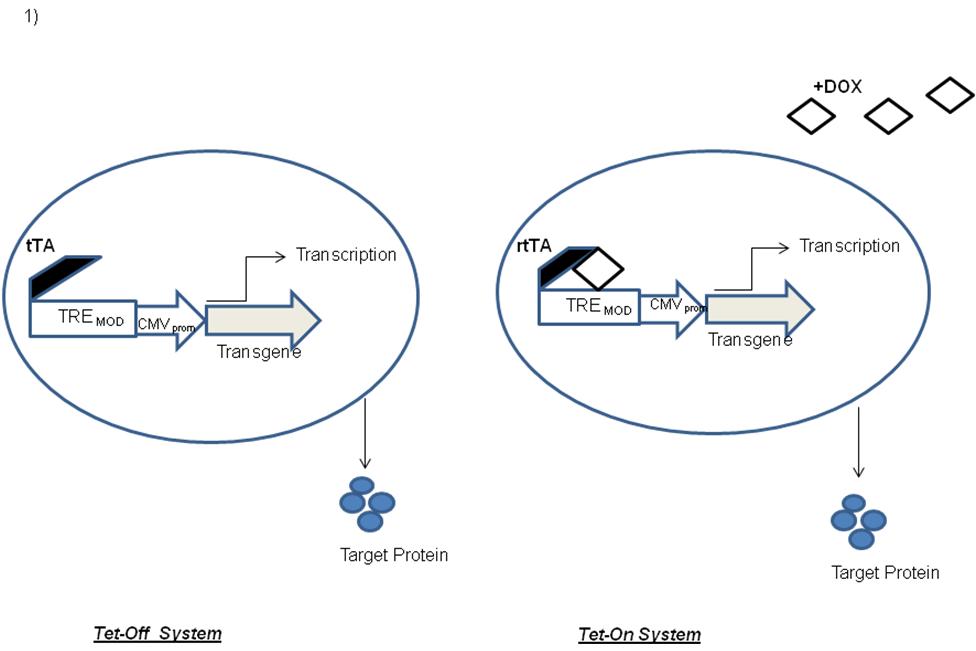
Figure 1 Induced expressions through the Tet-Off and Tet-On Systems. The Tet technology is based on the tetracycline-resistance operon of Escherichia coli and typically operates via the interaction of two core components, i.e. the Tet repressor protein (TetR) and the tetracycline-response element (TRE). In the Tet-off system, in the absence of doxycycline or tetracycline, the tTA would bind with high affinity to the tetO, activating transcription from the minimal CMV promoter. Tet-On requires tetracycline for binding to the tetracycline-responsive promoter that result in subsequent activation of the downstream genes.
Safety Issues of Lentiviral Vectors
Since the applicability of LVs has been developed from, in vitro studies to preclinical animal models and to corrective therapy in humans; it is most essential that lentiviral vector design and production rule out all hypothetical possibilities of risks. At most instances, a favorable outcome of gene therapy protocol invariably linked to a targeted transgene expression, in which the therapeutic gene is installed at an appropriate amount, in the right cells, and without developing toxicity or being eliminated by the immune system. Aiuti and co worker in 2009 pursued the long-term follow-up of 10 patients treated with stem cell gene therapy for severe combined immunodeficiency (SCID) using LVs (34), the results demonstrated significant success in reversal of the life-threatening immunodeficiency phenotype and a lack of the insertional mutagenesis that was associated with both the X-linked SCID (X-SCID)and chronic granulomatous disease (CGD)trials using MLV based retroviruses.
On the other hand, two major strategies are being used to minimize the possibilities of insertional mutagenesis: first, directing LVs insertion into specific sites within the host genome(35, 36), and secondly by exploring on integration deficient LVs to install therapeutic transgenes (Non integrating LVs; NILVs). Recently, Apoloina et.al. developed a variety of NILVs by introducing point mutations into the catalytic site, chromosome binding site, and viral DNA binding site of the viral integrase (IN) (37). In addition, they have mutated the IN attachment (att) sites within the HIV long terminal repeats (LTRs). All of the vectors produced show efficient reverse transcription and transgene expression in dividing cells and prolonged expression in non-dividing myotubes(37). Therefore it was demonstrated that NILVs have the potential to be used as tool for prolonged transgene expression in muscle in vivo.
To make LVs safer for use in the research and clinical setting, a significant modification into wild type genome has been the deletion of promoter and enhancer elements from the U3 region of the long terminal repeat (LTR); These are called self-inactivating LVs (SIN LVs)(38, 39). However, LVs oncogenic risks depends on several important variables including, the target cell type, viral copy number, the nature of the transgene, and the vector design (SIN versus non-SIN, choice of promoter/enhancers) itself. Cui et.al. (1999) were first to describe the concept of ‘minimal lentiviral vector’. There results showed that mutations in the major splice donor site (SD) markedly reduced viral RNA expression but had little effect on vector titer. Deletion of gag or env sequences, excluding RRE, led to a moderate reduction in vector titer. Further deletion of RRE slightly reduced viral RNA expression but markedly impaired vector function. Combined deletions of RRE, gag (except for the first 40 nucleotides), env, and the SD mutation resulted in a twofold increase in cytoplasmic viral RNA expression and a recovery of vector efficiency to approximately 50% of the wild-type level (40).
LVs for Biological Research
Since the first successful report of efficient LVs transduction of non dividing neurons by Nalidini et.al. in 1996(41), the field of LVs mediated gene delivery has recently been expanded to a wide spectrum of target tissues includes retinal or other sensory epithelium, neurons, cardiac or skeletal muscle, hepatocytes, endothelial cells, dendritic cells, hematopoietic stem cells, and dormant tumor stem cells(25, 42, 43). Numerous animal studies have now been undertaken with LVs and correction of disease models has been obtained. Recognizing the benefit of LVs for stable, long-term transgene expression in the central nervous system and in achieving cell-specific transgene expression in the brain have prompted numerous investigators to explore their use for the treatment of neurological diseases such as Parkinson's disease (PD), (HD), and motor neuron diseases (MNDs)(44, 45). Gene therapy is particularly appropriate for Parkinson disease (PD) since PD exclusively affects the dopaminergic neurons therefore the concept of in vivo DA replacement using gene therapy as a strategy for the treatment of patients with PD has been pursued for almost 2 decades. The initial attempts involve in vitro transduction of fibroblasts for transplantation into the DA depleted striatum and more recently, LVs were shown to be suitable to express small interfering RNA for treatment in models of Alzheimer's disease and amyotrophic lateral sclerosis. Thus a phase I–II clinical trial of intraputaminal delivery of Lenti-TH-AADC-GCH1 (three important genes necessary for dopamine synthesis) for PD has been initiated (44, 46, 47). In addition, several human gene therapy clinical trials are currently ongoing using LVs for treatment of a variety of human diseases namely β-thalassemia, ALD, and AIDS(48-50).
In the recent past, iPSc technology has emerged as one of the most promising technology to generate human embryonic stem (ES) cells and adult stem cells using LVs. James Thomson et al. demonstrated that the LVs mediated co-expression of a set of key factors (Oct4, Sox2, Nanog and Lin28) is highly successful in generating iPSc.(51). Thereafter numerous studies have demonstrated the clinical potential of LVs derived iPSc including generation of patient specific cells (52-54). Therefore the knowledge base of LVs mediated gene transfer is consistently expanding and hence it became possible to extend LVs use for plethora of biomedical and clinical applications.
Correspondance should be addressed to: Dr Pankaj Kumar MSc PhD, Environment Health Institute, BioPolis, Singapore. Phone:-+0065-84270638. E-Mail: panky8@yahoo.com
References:
1. Connolly JB. Lentiviruses in gene therapy clinical research. Gene Ther. 2002: 9:1730-1734.
2. Croyle MA, Callahan SM, Auricchio A, Schumer G, Linse KD, Wilson JM, et al. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J Virol. 2004: 78:912-921.
3. Hanawa H, Yamamoto M, Zhao H, Shimada T, Persons DA. Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus HS4 insulator element. Mol Ther. 2009: 17:667-674.
4. Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci U S A. 2006: 103:17372-17377.
5. Manilla P, Rebello T, Afable C, Lu X, Slepushkin V, Humeau LM, et al. Regulatory considerations for novel gene therapy products: a review of the process leading to the first clinical lentiviral vector. Hum Gene Ther. 2005: 16:17-25.
6. Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003: 21:2508-2518.
7. Chuang CK, Sung LY, Hwang SM, Lo WH, Chen HC, Hu YC. Baculovirus as a new gene delivery vector for stem cell engineering and bone tissue engineering. Gene Ther. 2007: 14:1417-1424.
8. Sasaki K, Chancellor MB, Goins WF, Phelan MW, Glorioso JC, de Groat WC, et al. Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes. 2004: 53:2723-2730.
9. Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000: 60:249-271.
10. Aghi MK, Chiocca EA. Phase ib trial of oncolytic herpes virus G207 shows safety of multiple injections and documents viral replication. Mol Ther. 2009: 17:8-9.
11. Georgopoulos LJ, Elgue G, Sanchez J, Dussupt V, Magotti P, Lambris JD, et al. Preclinical evaluation of innate immunity to baculovirus gene therapy vectors in whole human blood. Mol Immunol. 2009: 46:2911-2917.
12. King JC, Jr., Cox MM, Reisinger K, Hedrick J, Graham I, Patriarca P. Evaluation of the safety, reactogenicity and immunogenicity of FluBlok trivalent recombinant baculovirus-expressed hemagglutinin influenza vaccine administered intramuscularly to healthy children aged 6-59 months. Vaccine. 2009: 27:6589-6594.
13. Young A, McNeish IA. Oncolytic adenoviral gene therapy in ovarian cancer: why we are not wasting our time. Future Oncol. 2009: 5:339-357.
14. Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001: 7:33-40.
15. Ravet E, Lulka H, Gross F, Casteilla L, Buscail L, Cordelier P. Using lentiviral vectors for efficient pancreatic cancer gene therapy. Cancer Gene Ther. 2010: 17:315-324.
16. Huszthy PC, Giroglou T, Tsinkalovsky O, Euskirchen P, Skaftnesmo KO, Bjerkvig R, et al. Remission of invasive, cancer stem-like glioblastoma xenografts using lentiviral vector-mediated suicide gene therapy. PLoS One. 2009: 4:e6314.
17. Pawliuk R, Westerman KA, Fabry ME, Payen E, Tighe R, Bouhassira EE, et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001: 294:2368-2371.
18. Marwick C. FDA halts gene therapy trials after leukaemia case in France. BMJ. 2003: 326:181.
19. Howard DB, Powers K, Wang Y, Harvey BK. Tropism and toxicity of adeno-associated viral vector serotypes 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 2008: 372:24-34.
20. Latta-Mahieu M, Rolland M, Caillet C, Wang M, Kennel P, Mahfouz I, et al. Gene transfer of a chimeric trans-activator is immunogenic and results in short-lived transgene expression. Hum Gene Ther. 2002: 13:1611-1620.
21. Markusic DM, de Waart DR, Seppen J. Separating lentiviral vector injection and induction of gene expression in time, does not prevent an immune response to rtTA in rats. PLoS One. 2010: 5:e9974.
22. Raben N, Lu N, Nagaraju K, Rivera Y, Lee A, Yan B, et al. Conditional tissue-specific expression of the acid alpha-glucosidase (GAA) gene in the GAA knockout mice: implications for therapy. Hum Mol Genet. 2001: 10:2039-2047.
23. De Palma M, Venneri MA, Naldini L. In vivo targeting of tumor endothelial cells by systemic delivery of lentiviral vectors. Hum Gene Ther. 2003: 14:1193-1206.
24. van Hooijdonk LW, Ichwan M, Dijkmans TF, Schouten TG, de Backer MW, Adan RA, et al. Lentivirus-mediated transgene delivery to the hippocampus reveals sub-field specific differences in expression. BMC Neurosci. 2009: 10:2.
25. Toscano MG, Delgado M, Kong W, Martin F, Skarica M, Ganea D. Dendritic cells transduced with lentiviral vectors expressing VIP differentiate into VIP-secreting tolerogenic-like DCs. Mol Ther. 2010: 18:1035-1045.
26. Semple-Rowland SL, Eccles KS, Humberstone EJ. Targeted expression of two proteins in neural retina using self-inactivating, insulated lentiviral vectors carrying two internal independent promoters. Mol Vis. 2007: 13:2001-2011.
27. Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996: 93:11382-11388.
28. Ngan ES, Schillinger K, DeMayo F, Tsai SY. The mifepristone-inducible gene regulatory system in mouse models of disease and gene therapy. Semin Cell Dev Biol. 2002: 13:143-149.
29. Deuschle U, Meyer WK, Thiesen HJ. Tetracycline-reversible silencing of eukaryotic promoters. Mol Cell Biol. 1995: 15:1907-1914.
30. Baron U, Bujard H. Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol. 2000: 327:401-421.
31. Stieger K, Belbellaa B, Le Guiner C, Moullier P, Rolling F. In vivo gene regulation using tetracycline-regulatable systems. Adv Drug Deliv Rev. 2009: 61:527-541.
32. Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell. 2008: 2:151-159.
33. Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, et al. Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol. 2009: 1:46-54.
34. Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009: 360:447-458.
35. Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM. Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo. Proc Natl Acad Sci U S A. 2001: 98:11450-11455.
36. Du ZW, Hu BY, Ayala M, Sauer B, Zhang SC. Cre recombination-mediated cassette exchange for building versatile transgenic human embryonic stem cells lines. Stem Cells. 2009: 27:1032-1041.
37. Apolonia L, Waddington SN, Fernandes C, Ward NJ, Bouma G, Blundell MP, et al. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol Ther. 2007: 15:1947-1954.
38. Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998: 72:9873-9880.
39. Iwakuma T, Cui Y, Chang LJ. Self-inactivating lentiviral vectors with U3 and U5 modifications. Virology. 1999: 261:120-132.
40. Cui Y, Iwakuma T, Chang LJ. Contributions of viral splice sites and cis-regulatory elements to lentivirus vector function. J Virol. 1999: 73:6171-6176.
41. Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996: 272:263-267.
42. Trobridge GD, Wu RA, Beard BC, Chiu SY, Munoz NM, von Laer D, et al. Protection of stem cell-derived lymphocytes in a primate AIDS gene therapy model after in vivo selection. PLoS One. 2009: 4:e7693.
43. Watson DJ, Karolewski BA, Wolfe JH. Stable gene delivery to CNS cells using lentiviral vectors. Methods Mol Biol. 2004: 246:413-428.
44. Jarraya B, Boulet S, Ralph GS, Jan C, Bonvento G, Azzouz M, et al. Dopamine gene therapy for Parkinson's disease in a nonhuman primate without associated dyskinesia. Sci Transl Med. 2009: 1:2ra4.
45. Wong LF, Goodhead L, Prat C, Mitrophanous KA, Kingsman SM, Mazarakis ND. Lentivirus-mediated gene transfer to the central nervous system: therapeutic and research applications. Hum Gene Ther. 2006: 17:1-9.
46. Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003: 54:403-414.
47. Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001: 344:710-719.
48. Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009: 326:818-823.
49. Bank A, Dorazio R, Leboulch P. A phase I/II clinical trial of beta-globin gene therapy for beta-thalassemia. Ann N Y Acad Sci. 2005: 1054:308-316.
50. Boulad F, Riviere I, Sadelain M. Gene therapy for homozygous beta-thalassemia. Is it a reality? Hemoglobin. 2009: 33 Suppl 1:S188-196.
51. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007: 318:1917-1920.
52. Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008: 321:1218-1221.
53. Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009: 136:964-977.
54. Ebert AD, Yu J, Rose FF, Jr., Mattis VB, Lorson CL, Thomson JA, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009: 457:277-280.

































