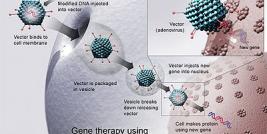
Integrating viral vectors hold great promise as gene transfer vectors for gene therapy purposes because they allow maintaining long-term expression of the therapeutic transgene throughout cell divisions. However, many issues related to integration of the provirus remain as a substantial risk for patients. The use of chromatin insulators has been proposed as a possible solution to problems raised by the integration of the vector.
Eukaryotic genome organization
Chromatin is the structure that the eukaryotic genome is packaged into, allowing considerable compaction of the DNA within the nucleus. This chromatin is composed of DNA and proteins, most of which are histones. The DNA-protein complex is the template for essential cell processes such as replication and transcription that are highly dependent on the precise chromatin conformation. The basic structural unit of the chromatin is the nucleosome composed of a DNA portion of approximately 145 base pairs wrapped around a histone octamer core. Each core histone contains two distinct domains: a histone fold motif within the nucleosome, and NH2 and COOH terminal tail domains projected away from the nucleosome core (1). Core histones in combination with other proteins (e.g. the histone H1, linking the nucleosomes together) are able to compact the DNA into more complex structures, often referred as higher order chromatin structures. The level of compaction of the DNA as well as local posttranslational modifications of the core histone tails (such as methylation, acetylation, phosphorylation…) defines distinct chromatin domains in which the accessibility of the DNA by the transcription cellular machinery is variable (2).
As a result, the expression status of eukaryotic genes is partly dependent on their location along chromosomes. Neighboring genes can be packaged together into a single chromatin domain acting as a functional unit in which the coordination of gene expression is ensured. These domains are defined by local modifications in the chromatin state and gene expression over the length of each domain is driven by regulatory elements such as promoters, enhancers and transcription repressors.
Euchromatin is the portion of the genome that is most permissive for gene transcription, in contrast to heterochromatin, which usually does not transcribe protein-coding genes. Either form of chromatin can be converted to the other, conversion to euchromatin being associated with new gene activation and conversion to heterochromatin being associated with new gene repression. Euchromatin is mildly condensed and rich in active enhancers able to activate genes over considerable distances whereas heterochromatin is composed of more compacted chromatin responsible for gene silencing and capable of encroaching on adjacent domains (see figure). It should be noted that the structure of the chromatin is still not fully understood and that alternative or intermediate states of chromatin may exist between hetero- and euchromatin.
Chromatin insulators
The ability of chromatin to organize functional autonomous units characterized by specific levels and patterns of expression is ensured through the establishment of boundaries that delimit these domains. In some cases, transcriptionally active genes may be embedded in an environment containing extensive regions of condensed chromatin capable of inappropriately silencing their expression. In other cases, signals from extraneous enhancers could cause incorrect pattern of expression of silent genes located nearby. Boundaries are responsible for ensuring the maintenance of the appropriate level of expression of each gene cluster by marking the barrier between chromatin domains of distinct states.
These boundaries might occur at various positions, as a result of a balance between countervailing processes (such as chromatin condensation and decondensation), or be fixed at specific DNA loci. Chromatin insulators are DNA elements that mark the boundaries of chromatin domains by limiting the range of action of enhancers and silencers and by preventing incursions of neighboring chromatin domains. Although they have a wide variation in DNA sequences and proteins that bind to them, they are characterized by at least one of the following abilities: enhancer-blocking or barrier activity (3, 4).
Enhancer-blockers
A chromatin insulator with enhancer-blocking properties is able to block the enhancer-promoter communication when positioned between them (5). Enhancer-blockers restrict the long-range activation potential of eukaryotic enhancers in order to strictly limit their influence to one or few specific target promoter.
Two models are proposed with regards to the mechanism of action of these insulators. The processive model envisions the relay of the enhancer signal to the promoter as a tracking action along the chromatin fiber. That model corresponds to a protein-tracking model in which the RNA-polymerase II-complex assembled at the enhancer tracks from the enhancer along the DNA to reach the promoter and activate mRNA synthesis. The transmission of this type of signal could be disrupted by the interposition of the insulator nucleoprotein complex interposed between the enhancer and the promoter. In the topological model, instead of sending a signal from afar, a distant enhancer has to be brought close to the promoter through mechanisms that allow direct interaction between the enhancer and the promoter (e.g. by loop formation). Insulators may recruit chromatin-modifying enzymes to locally change the chromatin state disabling loop formation and thus preventing the direct enhancer-promoter interaction (6).
Barrier elements
By acting as barriers, insulators may also prevent the spread of condensed chromatin able to silence expression, thus limiting the influence of the neighboring chromatin on transcriptionally active genes (7). Several mechanisms of barrier action have been proposed. Passive models propose the tethering of the barrier element to fixed structures in the cell nucleus or the creation of a nucleosome gap that could stop the propagation of silent chromatin. For example, matrix attachment regions (S/MAR) are involved in anchoring the chromatin to the nuclear scaffold resulting in the formation of chromatin loops (8). Active processes rely on the modification of the histones tails markers. Proteins that bind barrier elements have been shown to be able to recruit histone-modifying enzymes, locally competing with the spreading of silent chromatin markers (9).
Design of gene therapy vectors and improved safety
Major efforts have been made over the past two decades in order to capitalize on the ability of some viruses to permanently integrate within the cell genome for designing long-term gene therapy approaches. Indeed, the use of gene transfer vectors that have the ability to integrate a transgene with its regulatory sequences within the host cell genome ensures transmission of this genetic information to the target cell progeny. However, the expression of the transgene is still subjected to position-effect, i.e. highly dependent on the site of integration within host chromosomes: integration in heterochromatin usually leads to a silent state of the transgene whereas integration into a more permissive chromatin domain favors active transcription (although this often remains unnoticed given the propensity of viral vectors to integrate within or close active genes). In addition, the occurrence of serious adverse events refuted the assumption that activation of proto-oncogenes via replication-incompetent retroviral vectors was highly unlikely when vectors integrate only once or in a few chromosomal loci per cell.
One of the first successful trials of gene therapy had to face such adverse events. A g-retroviral vector was used to introduce a normal copy of the deficient gc cytokine receptor into autologous bone marrow hematopoeitic progenitor cells of young boys suffering from X-linked severe combined immunodeficiency. This treatment led to the rapid and complete immune reconstitution in 10 out of 11 boys. Unfortunately, 4 of them have developed T-cell proliferation similar to leukemia. In two cases, further studies have shown that there was integration-associated activation of the LMO2 proto-oncogene in leukemic cells attributed to the strong viral enhancer widespread influence at the site of integration (10, 11). The vector enhancer was able to transactivate the expression of neighboring genes at the site of integration of the vector, a phenomenon termed insertional mutagenesis.
Successful gene therapy does not only require therapeutic levels of expression of the transgene in a long-term manner, but also should not interfere with the endogenous gene regulation in the target cell type. In this regard, the prospect of implementing chromatin insulators into integrative gene transfer vectors is particularly promising. An effective element combining enhancer-blocking activity and barrier function would both limit the long-range influence of the transgene enhancer as well as protecting the transgene from silencing.
Some chromatin insulators have thus been implemented in vectors intended for gene therapy. The chicken b-globin insulator was incorporated in oncoretroviral vectors and was able to significantly reduce position effects (12, 13). Retroviral vectors designed to contain the sea urchin sns5 insulator also displayed better shielding against silencing (14). However, chromatin insulators are not always compatible with the viral vectors biology. They rarely fit with the limited carrying capacity of the vectors, they are often responsible for titer decrease and even transgene expression decrease, and they may prevent proper transduction of target cells, limiting their widespread use (15).
Perspectives
In order to improve the risk-benefit ratio associated with retroviral-mediated gene therapy, several approaches are currently developed, like the design of self-inactivating vectors containing an internal promoter to drive transgene expression instead of the long terminal repeat-containing enhancer of standard retroviral and lentiviral vectors. These vectors have shown improved safety features but they may still keep residual capacity to alter gene regulation at the site of integration and are not freed from possible position-effect. Therefore, identifying or designing new chromatin insulators that could be implemented within gene therapy vectors may definitely contribute to improve the safety as well as the efficacy of such therapeutic strategies. Through their barrier activity, they may allow the use of weaker or tissue-specific promoters that failed so far to compete with transgene silencing once integrated in the genome and thus to provide long-term transgene expression. They also raise the possibility of safer therapeutic approaches thanks to the enhancer-blocking property that would limit the influence of the vector on the host cell. Although highly desirable, these elements will not fully abolish any risk linked to vector integration, such as insertional inactivation of a gene or formation of gene fusions, and other aspects of vector toxicology still remain to be explored.
Schematic illustration of eukaryotic genome organization.
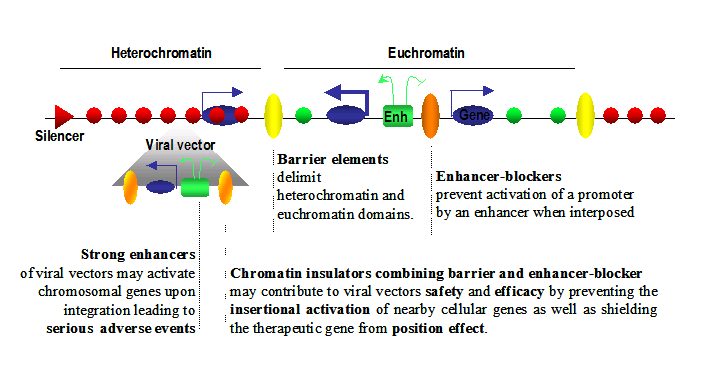
Legend: Eukaryotic genomes are composed of chromatin harboring different states. Heterochromatin corresponds to closed chromatin, non permissive for gene expression, highly dense in nucleosomes bearing markers of silent chromatin (red circles). In this domain, silencers (red triangles) are responsible for limiting genes’ transcription level (thin blue arrows). On the other hand, euchromatin domains are composed of nucleosomes bearing marks of permissive chromatin (green circles) and contain highly expressed genes (thick blue arrows). Enhancers (green squares) are driving the expression of these genes and are capable of activating genes over large distances (green arrows). Chromatin insulators secure the delimitation of chromatin domains by limiting the spread of silent chromatin (barrier element, yellow circles) and also restrict the long-range influence of enhancers (truncated green arrow) when interposed between them and promoters (enhancer-blocker element, orange circle). Viral vectors (grey triangle) flanked by effective chromatin insulators, combining both enhancer blocking and barrier properties, raise the prospect of safer and more efficient gene therapy vectors.
Armelle Gaussin and Nic Mermod work in the Laboratory of Molecular Biotechnology, UNIL, Switzerland.