Article by: 370
A number of different enzymes and molecules are involved in the maintenance of reduction-oxidation reaction (redox) balance in tissues. The three mammalian dismutases, cytosolic CuZnSOD (SOD1), mitochondrial MnSOD (SOD2), and extracellular superoxide dismutase (SOD3) are among the most important redox enzymes. They differ by the cellular localization and therefore have slightly different regulation of expression and therapeutic effects in tissue damage recovery suggesting distinct targets for gene therapy. In the current review I focus on the regulation of SOD3 gene expression in tissues and on the effect of SOD3 transgene on signal transduction.
1. SOD3-mediated response is caused by controlled production of hydrogen peroxide
SOD3 is a tetrameric extracellular enzyme that is able to bind reversibly to cell surface structures by the carboxyterminal heparan sulphate binding domain. Cell surface bound extracellular superoxide dismutase (SOD3), which dismutases superoxide (O2.-) to hydrogen peroxide (H2O2), is generally considered as an antioxidative enzyme even though both the substrate and the reaction end product are reactive oxygen species (ROS) being able to cause destructive effects in the cellular environment. High concentration of H2O2 causes apoptosis and in the presence of free iron excess H2O2 can spontaneously lead to formation of hydroxyl radical (OH•) that is one of the most reactive and deleterious ROS. It is therefore interesting that sod3 gene transfer has such a strong therapeutic response in tissue damages increasing cell proliferation and attenuating inflammation (1,2,3) suggesting that balance between O2.- and H2O2 is essential for tissue injury recovery and for normal cellular functions. The therapeutic response even after adenovirus gene transfer suggests strong regulation of the SOD3 transgene expression to avoid production of toxic levels H2O2.
2. Regulation of SOD3 expression
Catalase, which neutralizes H2O2 into oxygen and water, is an intracellular enzyme expressed in peroxisomes and is therefore unable to metabolize extracellular SOD3-derived H2O2 and has minuscule effect on SOD3-mediated signal transduction (2). Since so far there are no reports suggesting extracellular catalase activity specifically neutralizing SOD3 derived H2O2, there is an obvious need for the cells to control the excess production of H2O2 at their cell membranes. According to the recent reports both endogenous and exogenous expression of SOD3 is regulated at several levels; 1) Pre-transcriptionally: we have recently reported that Ras-Erk1/2 mitogen pathway is one of the main signal transduction pathways in SOD3 production (2), which may partly explain the expressional differences between tissues. We have also previously demonstrated the presence of GpG islands located on the coding sequence and at the vicinity of sod3 gene. According to our data high methylation level was decreased in cardiovascular endothelial hyperplasia suggesting that epigenetic modification of sod3 gene could potentially form another pre-transcriptional regulatory machinery in proliferative diseases (4); 2) Post-transcriptionally: we have further shown in vitro that only a part of the mRNA produced after adenovirus gene transfer is translated into protein (5) suggesting a strong post-transcriptional regulatory step; 3) Post-translationally inside the cell: we and others have reported secretion of partially C-terminally truncated SOD3 with decreased affinity to cellular membranes increasing the biodistribution of the secreted enzyme (6,7). Another significant intracellular event in SOD3 secretion pathway is the modification of disulphide bridge structures resulting in either active or inactive SOD3 (8). In general, H2O2 is able to diffuse through cell membranes yielding approximately 10-fold difference between the extracellular and intracellular concentrations. According to recent report H2O2 can directly modulate protein tyrosine phosphatase activity by oxidizing cystein residues (9) suggesting that the rearrangement of disulphide bridges could represent yet another post-translational regulatory step for SOD3 in the presence of increased extracellular H2O2; 4) Post-translationally after secretion of the enzyme: after secretion of the enzyme it binds on the cell surface structures by C-terminal domain, which contains protease sensitive site. After degradation of the C-terminal end continuously active SOD3 becomes soluble, which increases the biodistribution of the enzyme simultaneously decreasing the local H2O2 production (10,11,12). All these regulatory steps ensure that local SOD3 concentration in tissues and consequently H2O2 levels stay within physiological frames aiming to recover and to stabilize the normal tissue functions.
3. SOD3 based therapy in cardiovascular injuries
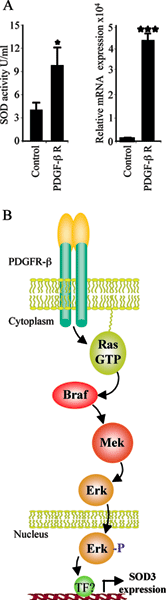
Figure legend. PDGF-b receptor activated SOD3 production. Pdgf-b receptor (5mg) was transiently transfected into HEK 293T cells with polyfect (Qiagen). The expression analysis was done 48 hours after transfection. The transient transfection of pdgf-b receptor increased both mRNA synthesis (p<0.001) and SOD total protein activity (p<0.05)(2) in the cell culture medium suggesting cell membrane receptor mediated regulation of SOD3 production. B. Cell membrane PDGF-b receptor signaling and consequent activation of mitogen signal transduction pathway mediate SOD3 production. Translocation of phosphorylated Erk into the nucleus potentially stimulates transcription factors needed for increased SOD3 gene expression. The involvement of mitogen signal transduction indicates the importance of the enzyme in cell proliferative disorders, e.g. in the cardiovascular endothelial hyperplasia, which could be one the main physiological functions of the enzyme.
Due to stringent regulation of gene expression the stimulation of endogenous SOD3 expression is limited to 3-5-fold increases (13,14,15). In cardiovascular tissue injuries, especially in restenosis damage, SOD3 expression is decreased with simultaneous increased superoxide production by NADPH oxidase gene family creating a redox unbalance with aberrant synthesis of different ROS (16,17,18,19). The reasons causing decreased SOD3 production in the injury are not well characterized but recently it has been shown that TNFa-p38MAPK pathway efficiently decreases SOD3 production (13, 20, 21). Since TNFa is highly upregulated in restenosis (22) and there is an immediate long-term activation of p38MAPK in cardiovascular damage (23, 24), this pathway could potentially explain the decreased SOD3 production seen in in vivoinjury models.
The temporal redox unbalance in cardiovascular damages creates a foundation for sod3 gene therapy, which has been shown to result in significant improvements in the injury recovery by increasing endothelium recovery after balloon angioplasty, by increasing tissue glucose metabolism and cell proliferation in hind limb ischemia, and by attenuating cardiac muscle damage in ischemic injury (1, 2, 25,26,27,28). The mechanism explaining the increased recovery is partially due to newly balanced redox system, reduced inflammatory cell migration, and increased endothelial cell proliferation in the case of cardiovascular damage. Recently we have shown that adenoviral SOD3 gene transfer is able to stimulate Ras-Erk mitogen signal transduction pathway leading to increased AP1 and CREB transcription factor activation, increased vegf growth factor expression, increased cell proliferation, and improved tissue metabolic performance (2). Our data further demonstrated a positive regulatory loop for SOD3 expression showing mitogen pathway derived increase in the endogenous SOD3 production suggesting signal transduction self-regulation for the production of the enzyme. The cell membrane receptors responding to the tissue’s need for SOD3 synthesis are not identified. Since Ras-Erk signal transduction pathway is responsible for sod3 mRNA synthesis it is likely that cell membrane receptors upstream of Ras mediate the extracellular signaling leading to increased enzyme production. In our current experiment transient transfection of pdgf-b receptor, which is upstream of mitogen pathway and frequently involvement in cell proliferative disorders, into HEK 293T cells increased sod3 mRNA synthesis and total SOD activity in vitro (Fig 1).
The data showing that only 2-3 fold increase in active SOD3 concentration (2) is able to enhance tissue injury recovery questions the need for high overexpression of the enzyme. The multi-step regulation of SOD3 production ensures the non-toxicity of sod3 based gene therapies whether they aim to increase endogenous gene expression or are meant to restore the temporal decrease of SOD3 by gene transfer. The stringent regulation of SOD3 production on the other hand reduces the need for pharmacological estimation of therapeutic dose of the transferred gene. All in all, since in the redox gene therapy the healing can be caused also by other factors than the overexpression of the transgene, the identification of the regulatory network at the level of signal transduction mediating the therapeutic effects is vitally important to enhance the transgene-derived correction of the target tissue injury.

































