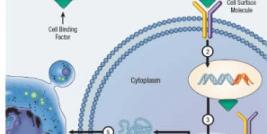
Ceregene Announces Clinical Data from Phase 2 Clinical Trial of CERE-120 for Parkinson's Disease
San Diego, CA - November 26, 2008 – Ceregene, Inc., a biopharmaceutical company, today reported clinical data from a double-blind, controlled Phase 2 trial of CERE-120 in 58 patients with advanced Parkinson’s disease. The trial did not demonstrate an appreciable difference between patients treated with CERE-120 versus those in the control group. Both groups showed an approximate 7 point improvement in the protocol-defined primary endpoint (Unified Parkinson’s Disease Rating Scale- motor off score at 12 months), relative to a mean at baseline of approximately 39 points.