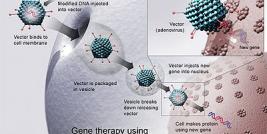
Gene Therapy for Duchenne Muscular Dystrophy
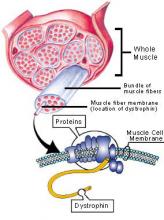
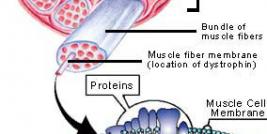
Duchenne muscular dystrophy (DMD) is an X-linked genetic disease characterized by the absence of dystrophin in the muscle. This large protein of 427 kDa is encoded by a 14 kb mRNA (79 exons) (1). This dystrophin protein is located under the membrane of the muscle fiber and interacts with other trans-membrane proteins. It is needed to insure mechanical stress resistance during muscle contraction. The lack of dystrophin weakens the sarcolemma and thus makes fibers less resistant to stress. When a fiber is damaged, the satellite cells (stem cells located near the muscle fibers) are activated and proliferate to allow fiber regeneration (2). In the case of a DMD patient, fibers are frequently damaged due to the lack of dystrophin. The regeneration is also ensured by the proliferation of satellite cells though their number decreases rapidly and when there are no more satellite cells, the average diameter and the number of fibers progressively decrease bringing the development of fibrosis and fat infiltrations into the muscles. There is currently no treatment for DMD and life expectancy is between 20 to 25 years of age.