A strategy that combines gene therapy with blood stem cell therapy may be a useful tool for treating a fatal brain disease, French researchers have found. These findings appear in the 6 November 2009 issue of the journal Science, which is published by AAAS, the nonprofit science society.
In a pilot study of two patients monitored for two years, an international team of researchers slowed the onset of the debilitating brain disease X-linked adrenoleukodystrophy (ALD) using a lentiviral vector to introduce a therapeutic gene into patient's blood cells. Although studies with larger cohorts of patients are needed, these results suggest that gene therapy with lentiviral vectors, which are derived from disabled versions of human immunodeficiency virus (HIV), could potentially become instrumental in treating a broad range of human disorders.
"This is the first time we were able to successfully use an HIV-derived lentivirus vector for gene therapy in humans, and also the first time that a very severe brain disease has been treated with efficacy by gene therapy. We've demonstrated that this HIV-derived lentivirus vector works as was hoped for so many years," said coauthor Patrick Aubourg, professor of pediatrics at University Paris-Descartes and head of a research unit at Inserm-University Paris Descartes.
Featured in the movie "Lorenzo's Oil," ALD is a severe hereditary condition caused by a deficiency of a protein called ALD that is involved in fatty acid degradation. Sufferers steadily lose their myelin sheath, the protective layer that coats nerve fibers in the brain. Without myelin the nerves lose function, leading to increasing physical and mental disability in patients. X-linked ALD, the most common form of the disease, affects boys starting at age 6-8 years of age and death usually occurs before the patients reach adolescence.
Bone marrow transplants typically slow progression of the disease because the donor marrow includes cells that develop into myelin-producing cells. However, finding a matching bone marrow donor can be a challenging and lengthy process, and the procedure carries considerable risks.
Genetically correcting the blood stem cells in the patients' own bone marrow may prove to be a valuable alternative approach when no matched donors are available.
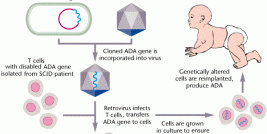
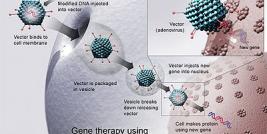
In most gene therapy studies, a working gene is inserted into the genome to replace a dysfunctional, disease-causing gene. A carrier molecule called a vector is used to deliver the therapeutic gene into the patient's cells. Vectors are typically the backbones of viruses that have been genetically altered to carry normal human DNA. Scientists have recently turned to vectors based on the lentivirus genus of retroviruses, which includes HIV. Lentiviral vectors are a type of retrovirus that can infect both dividing and nondividing cells, and are thought to provide long-term and stable gene expression, unlike other retroviruses.
"The HIV-derived lentivirus vector allows expression of the therapeutic gene in principle for life, because the therapeutic gene is inserted in the chromosomes—the genome. Therefore, cells that derive from the initially corrected cells, stem cells in particular, will continue to express the therapeutic gene forever," said Aubourg.
In the study, blood stem cells were removed from the patients and genetically corrected in the lab, using a lentiviral vector to introduce a working copy of the ALD gene into the cells. The modified cells were then infused back into the patients' after they had received a treatment that destroyed their bone marrow. Two years later, healthy ALD proteins were still detectable in both patients' blood cells. Encouragingly, both patients showed neurological improvement and a delay in disease progression comparable to that seen with bone marrow transplants.
The healthy ALD protein was expressed in about 15 percent of blood cells, yet surprisingly this low level was sufficient to slow brain disease in ALD. "This percentage of correction will not be sufficient for all diseases," warns Aubourg. "There is a lot of work to be done to make this gene therapy vector more powerful, less complicated, and less expensive. This is only the beginning," he said.
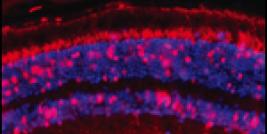
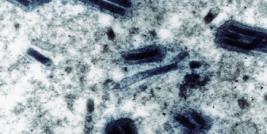
Figure Legend. This figure represents four cells, four purified CD34+ (the CD34+ cell population comprises true hematopoetic stem cell) from patient P1. These cells were sampled/purifed from his bone marrow 2 years after gene therapy. The blue circles represent nucleus of cells. The red dots in one cell are peroxisomes (intracellular organelle) in which the ALD protein encoded by the integrated lentiviral vector is expressed. In other cells, the red dots are not detectable because these cells are not corrected after gene therapy and the mutation of ABCD1 gene in this patient results in complete absence of ALD protein expression in peroxisomes. The exact ratio of corrected/non-corrected CD34% cells in patient P1, two years after gene therapy, is 18 percent. (Image courtesy of Patrick Aubourg).
Gene therapy is not without serious risks. Like other retrovirus vectors, the HIV-derived lentivirus vector is tasked with inserting the therapeutic gene in the chromosomes of the patients' cells. In a worst case scenario, this action could disturb the biology of the cells and patients could end up with leukemia; this outcome has occurred in past gene therapy trials. "The HIV-derived lentivirus vector basically has this same risk, although the design of the vector makes patients less prone to this side effect," said Aubourg.
This research was funded by INSERM (National Institute of Health and Research Medical), Assistance Publique des Hôpitaux de Paris, PHRC programs, the Deutsche Forchungsgemeinschaft and the German Ministry of Education and Research, the European Leukodystrophy Association, the Association Française contre les Myopathies, the Stop ALD Foundation and University Paris-Descartes.
Source: Eureka Alert (AAAS).