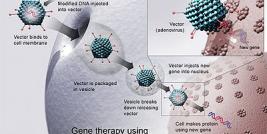
Gene therapy suffered another blow, when Dutch biotech company Amsterdam Molecular Therapeutics, AMT, got another negative opinion for it's lead drug Glybera. The opinion was already a second for Glybera, since the company got first negative opinion in July 2011 and asked for re-examination from EMA.
Interestingly, this time the company got a positive opinion from CAT, Committee of Advanced Therapies, which might indicate that the product was deemed safe and effective, but CHMP, The Committee for Medicinal Products for Human Use, voted to yield a negative decision.
"The CHMP Rapporteurs, SAG and the CAT concluded that data from three Glybera clinical trials demonstrated meaningful evidence of clinical efficacy, without any major safety concerns." States AMT on their press release.
The CHMP grounds for the dismissal were as before, risk/benefit ratio was not approvable fro Glybera.
Following the decision, AMT's stock rate dropped from 0.7 to 0.3. The company announced that they will pursue with a new strategy.
"We are encouraged by the validation provided by the CHMP Rapporteurs, Scientific Advisory Group and the Committee for Advanced Therapies regarding our gene therapy platform, indicating that safety is not an issue,” said Jörn Aldag, CEO of AMT.
It remains to be seen, when another gene therapy company will attempt to gain the marketing approval from EMA.