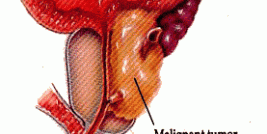
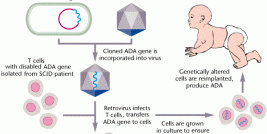
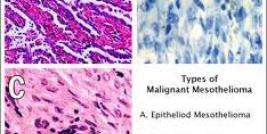
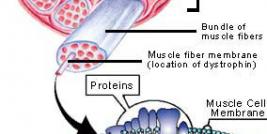
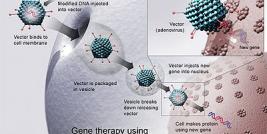
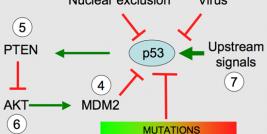
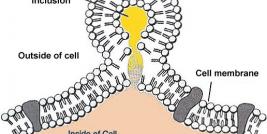
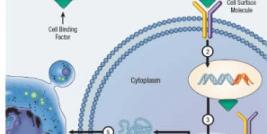
Diamond Biopharm is one of very few European regulatory companies which has experience in clinical and regulatory development programmes within the gene therapy sector, to an advanced stage of development. Its first European Marketing Authorisation Application submission for a gene therapy product will be in 2009.
The company was founded in 2005, by Maureen Graham. Formerly Director of European Regulatory Affairs at Amgen, Maureen has a huge amount of experience in the biotechnology field and was later engaged (as Diamond BioPharm Limited) to lead the regulatory development of a pioneering gene therapy treatment. Supported by an experienced team of regulatory experts, Diamond is now a leader in this field.
“Bridging the Gap … from invention to the market place”
What can Diamond BioPharm do for your Gene Therapy programme?
- Bring expertise and experience through direct project involvement
- Prepare high quality documentation to support Clinical Trial Applications (IMPD, IND, CTA)
- Links with North American regulatory specialists to support global development programme
- Manage regulatory dossier life-cycle from project initiation to submission, MA approval and beyond
- Assist with writing, editing and reviewing Modules 3, 4 and 5 of the CTD dossier
- eCTD-ready documentation and publishing
- Act as legal representative in the EU for clinical trials
- Be the central point for communication with regulatory agencies
- SME status means lower fees for Scientific Advice meetings and regulatory submission fees
Critical issues that Diamond BioPharm can assess and add value to the product development include:
- Comparability studies for changes in production processes
- Characterisation studies
- Viral safety evaluation
- Non-clinical …choice of animal models
- Germ line transmission
- Integration
- Environmental risk
- Clinical protocol design
- Risk management planning
- Pharmacovigilance
Examples of Diamond BioPharm Limited’s major successes
- Orphan Drug designation approvals
- Approval of Paediatric Investigation Plan
- Scientific Advice and Product Strategy meetings
- Successful Clinical Trial Applications in more than 20 countries
- 10+ Centralised Marketing Authorisation approvals
Diamond Pharma Services is a group of companies which provides technical support to the pharmaceutical industry, focusing on specialised services such as Regulatory, Quality and Drug Safety.
Our emphasis is on the following areas:
- Regulatory Affairs
- From Product Concept to Registration and Beyond Product Development
- Nonclinical, CMC and Clinical Aspects Pharmacovigilance: Clinical trial (Phase I-IV), Post-Marketing and QPPV Services Compliance
- GLP, GMP, GCP and QP Services Patient Information Leaflet Testing
- Over 120 Tested with 100% Positive Agency Feedback
- Our clients range from virtual start-up companies to the largest multi-national corporations.
- Our expertise includes advanced therapy medicinal products (gene therapy products), products of recombinant DNA and hybridoma technology, nucleotide based products (RNA and DNA), synthetic peptides, chemical entities (innovative and generic), vaccines (therapeutic and prophylactic) and blood products.
Diamond Pharma Services we have a network throughout Europe hence our ability to provide a local solution on the ground in a specific EU country when required. Diamond Pharma Services has also built strong relationships with other groups outside of Europe to expand the geographical expertise and services that are offered to clients of the Diamond group. Established and proven relationships are in place to offer complete solutions in the USA, Canada, Australia and New Zealand.
Paul Cronin is the Director of Business Development for Diamond Pharma Services. Paul Cronin has a BSc in Biochemistry and an MSc in international business and management. Paul previously headed business development at the Smerud Medical Research Group which is a clinical Contract Research Organisation operating in the European area with head office in Norway and subsidiary wholly owned offices throughout Europe including Austria, Denmark, Finland, Hungary, Sweden, Poland, Russia, United Kingdom. Paul started his pharmaceutical career as a regulatory affairs consultant at ERA Consulting. During his time at ERA Consulting he was involved in evolving this into a leading international regulatory consultancy and was responsible for all of its global business development activities. Paul has overall responsibility for the development, direction and implementation of Diamond Pharma’s global marketing and business development strategy.